Streamline Regulatory Writing with AI-Powered Solutions
Pharmaceutical regulatory writing is a complex, time-intensive process involving the meticulous compilation, review, and formatting of critical documents. Mistakes in this critical process can lead to significant delays in drug approvals, ultimately affecting patient care. The pharmaceutical industry is experiencing a paradigm shift in how regulatory documents are created, reviewed, and submitted. Certara's CoAuthor™ is innovative GenAI software for regulatory and medical writing, designed to streamline the drafting of regulatory documents, representing a breakthrough that could fundamentally change how regulatory teams approach their most critical work.

AI offers transformative potential that can be applied effectively in regulatory writing. It requires beyond everyday AI applications’ basics, an industry-specific approach to the solution. Regulatory documentation’s complexity and high stakes demand tailored AI solutions that ensure accuracy while enabling efficiency.
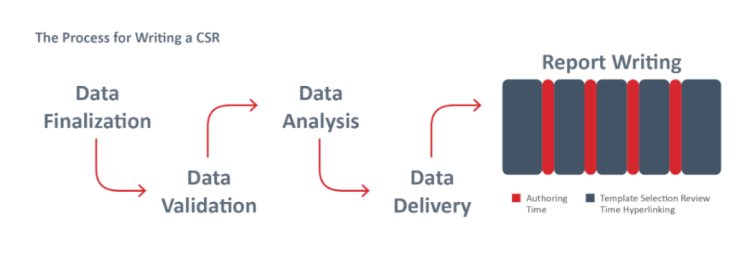
CoAuthor™ is a cutting-edge generative AI platform purpose-built for regulatory and medical writing. Designed to enhance the efficiency and accuracy of document creation, CoAuthor leverages an advanced biomedical GPT, an extensive eCTD-compliant template library, and structured content authoring capabilities. This empowers medical writers to focus on interpreting data and crafting meaningful messages, rather than getting bogged down by formatting and repetition.
Integrated directly with Microsoft Word, this specialized GenAI software for medical writing ensures secure, organization-specific use, eliminating data leakage risks and enhancing document quality. The built-in tools for transparency, collaboration, and standardization help streamline the submission process, ultimately accelerating the access to life-saving medicines for patients.
Core Capabilities That Set CoAuthor Apart
Advanced Document Generation: Generate documents such as patient narratives, clinical study reports, and protocols with ease. CoAuthor understands the nuanced requirements of regulatory documentation, ensuring that every generated document meets industry standards and regulatory expectations.
Structured Content Architecture: Leverage structured content authoring for content reuse and consistency, enabling teams to build a robust foundation of reusable components that maintain consistency across all regulatory submissions.
Intelligent Automation Automate formatting, hyperlinking, and metadata population for efficiency, eliminating the tedious manual work that traditionally consumed valuable time and introduced potential errors.
The CoAuthor Advantage
CoAuthor distinguishes itself through a comprehensive suite of features specifically designed for regulatory writing workflows:
-
Regulatory Document Template Suite: Pre-built templates aligned with eCTD requirements
-
Structured Content Authoring: Ensures consistency and enables intelligent content reuse
-
Regulatory-Specific Generative AI: Purpose-built AI trained for pharmaceutical and medical writing contexts
-
Auto Styling & Hyperlinking: Automatic formatting that meets regulatory standards
-
Traceability & Version Control: Complete audit trails for regulatory compliance
-
Real-Time Preview: Instant visualization of document formatting and structure
-
Collaborative Authoring & Review: Seamless teamwork capabilities
-
Metadata & Document Repository: Centralized management of all regulatory documents
-
Off-the-Shelf Prompt Library: Pre-configured prompts optimized for regulatory writing tasks
-
Microsoft Word Integration: Works within familiar environments without workflow disruption
As the pharmaceutical industry continues to evolve, tools like CoAuthor represent the future of regulatory documentation. By combining cutting-edge AI with deep regulatory expertise, CoAuthor enables teams to work more efficiently, produce higher-quality documents, and ultimately contribute to faster patient access to life-saving therapies.
The question isn't whether AI will transform regulatory writing—it's whether your organization will lead this transformation or follow behind. CoAuthor offers the opportunity to be at the forefront of this evolution, delivering tangible benefits today while building capabilities for tomorrow's challenges.
Ready to experience the future of regulatory writing? Schedule a demonstration to see how CoAuthor can transform your regulatory documentation processes and accelerate your path to market.