Vaccines and Drugs under clinical trials for COVID 19
The coronavirus disease (COVID-19) virus is spreading rapidly, and scientists are endeavouring to discover drugs for its efficacious treatment in China.

The coronavirus disease (COVID-19) virus is spreading rapidly, and scientists are endeavouring to discover drugs for its efficacious treatment in China. Chloroquine phosphate, an old drug for treatment of malaria, is shown to have apparent efficacy and acceptable safety against COVID-19 associated pneumonia in multicentre clinical trials conducted in China. The drug is recommended to be included in the next version of the Guidelines for the Prevention, Diagnosis, and Treatment of Pneumonia Caused by COVID-19. Chloroquine has so far showed no obvious serious adverse reactions in the more than 100 participants in the trials.
China has commenced a Phase I clinical trial of a vaccine against Covid-19, the infection caused by the novel coronavirus. The trial is designed to enrol 108 volunteers aged 18–60 years. Participants will be divided into three groups and administered with different dosages.
The first US clinical trial of a Covid-19 vaccine candidate, which is Moderna’s mRNA-1273, has started at Kaiser Permanente Washington Health Research Institute (KPWHRI) in Seattle. Funded by the National Institutes of Health (NIH)’s National Institute of Allergy and Infectious Diseases (NIAID), the trial has dosed its first participant.
mRNA-1273 is an mRNA vaccine designed to target SARS-CoV-2 encoding a prefusion stabilised form of the Spike (S) protein. Moderna selected the candidate in alliance with the Vaccine Research Centre (VRC) at the NIAID.
The Coalition for Epidemic Preparedness Innovations (CEPI) funding supported the production of the first clinical batch.
During the Phase I trial, the safety and immunogenicity of 25μg, 100μg, 250μg dose levels of mRNA-1273 given on two-dose vaccination schedule 28 days apart will be assessed in a total of 45 healthy adults aged 18 to 55.
Japan to trial HIV medication and Ebola drugs on COVID-19 Patients
Japan chief cabinet secretary plans to conduct clinical trials of an existing HIV medication for the treatment of Covid-19.
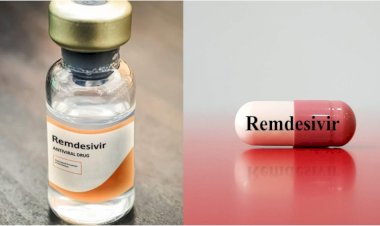
The National Centre for Global Health and Medicine (NCGM) supposed that it will join an international clinical trial on the use of remedesivir developed as a medicine for Ebola, to treat people infected with the new COVID.
In U.S. National Institute of Health Clinical trial, the remdesivir will be administered to be some pneumonia patients caused by new COVID.
The NCGM also done clinical research on existing drugs like Alvesco used to treat with asthma, is expected to be tested on coronavirus patients without pneumonia.
Meanwhile, Japanese Biopharmaceutical firm Agnes Inc. supposed that it and Osaka University had completed development of a DNA vaccine against the new COVID and that it would begin testing it in animals.
https://www.japantimes.co.jp/news/2020/02/22/national/science-health/flu-drug-avigan-coronavirus/#.Xn11R4gzbIU
https://www.clinicaltrialsarena.com/news/coronavirus-covid-19-choroquine-data/
https://www.clinicaltrialsarena.com/news/nih-gilead-remdesivir-monkey-data/
https://www.clinicaltrialsarena.com/news/china-approves-favilavir-covid-19/
https://www.clinicaltrialsarena.com/analysis/coronavirus-mers-cov-drugs/

 Meghana
Meghana